
Gold nanoparticles help Japanese sake arrive internationally with its fresh taste.
Nov. 15, 2018

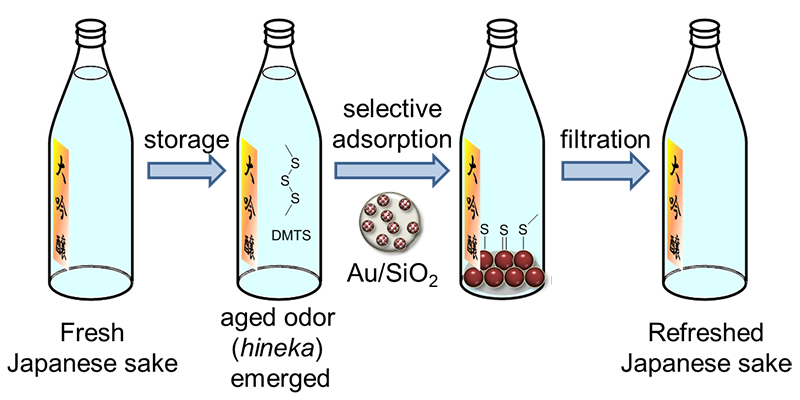
Japanese sake, a traditional alcoholic drink made from the fermentation of rice, has recently become more famous, and is being exported to many countries. The mellow and smooth flavor attracts many people and much like wines derived from grapes, Japanese sake can be married to a variety of foods. However, it is difficult to keep the fresh taste Japanese sake is known for once the bottle has been opened for a long period of time. The sake tends to develop an unpleasant odor once left open, which may have inhibited its adoption internationally. The organic compound DMTS (1,3-dimenthyltrisulfane) within the sake is considered to be the cause of the odor.
A Japanese research group led by Makoto Tokunaga, Professor of the Faculty of Science, at Kyushu University, has discovered a method to selectively adsorb DMTS with gold (Au) nanoparticles (NPs).
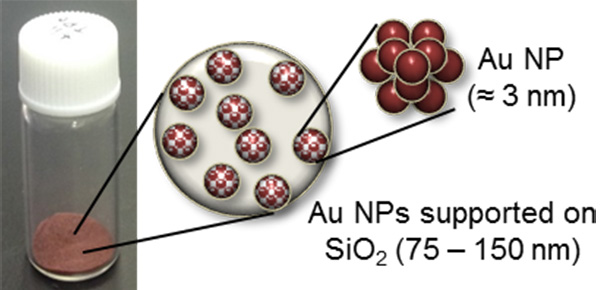
“Firstly, we developed a preparation method of small gold NPs supported on SiO2 (Au/SiO2). Small gold NPs supported on TiO2 are well known to have excellent catalytic activity. But it was difficult to deposit small gold NPs on various metal oxide including SiO2. Au–amino acid complexes, chloride-free and water-soluble precursor, were successful for preparing small gold NPs on SiO2. Au/SiO2 selectively adsorbs DMTS without a decrease in the concentration of other components such as ester, which makes “ginjoka”. On the other hand, activated carbon, conventional adsorbent, reduces both DMTS and esters concentration.” said Professor Tokunaga.
Haruno Murayama, Associate Professor of Tokunaga laboratory, said “Several noble metals other than gold also adsorb DMTS and other sulfur compounds. We will elucidate the difference of adsorption style and strength for each metal. We expect gold only adsorbs DMTS, but some metals work as a catalyst as well as an adsorbent. In addition, some sulfur compounds such as 3-MH (3-mercaptohexan-1-ol), 3-MHA (3-mercaptohex-1-yl acetate), and 4-MMP (4-mercapto-4-methylpentan-2-one) bring positive flavors for wines and other beverages. Selective desulfurization leaving these compounds are also challenging. We will also make efforts for the practical use of Au/SiO2 system.”
The discovery will contribute to Japanese sake being enjoyed around the world with the fresh taste it is known for. The research was published in Scientific Reports on October. 30, 2018.