
A molecular spring behind the cohesive behavior of cancer cell migration
Jun. 27, 2018
The team of researchers led by Professor Junichi Ikenouchi at the Faculty of Science, Kyushu University have uncovered the mechanism that allows a group of cells to move as a cohesive unit, such as in cancer. Published in Cell Reports , the study details how elevated tension, or ‘pulling force’, between cells alters the protein composition of the cell adhesion complex and triggers biochemical changes within the cell to enable cohesive behavior of the epithelial cell sheet.

The integrity of our body is preserved from the outside environment by a specialized type of cell called the epithelial cell. By forming a continuous cell sheet, epithelial cells can at once repel infectious agents like bacteria and allow the selective passage of life-sustaining nutrients, other small molecules and ions. Epithelial cells do so by interlocking proteins at the cell surface between neighboring cells to form structures called cell adhesions. In the past, the breakdown of these cell adhesions was thought to underlie the metastatic and invasive behavior of cancer cells originating from epithelial tissues. However, recent reports show that this is not always the case and that some cancers of epithelial origin can maintain their sheet organization while metastasizing and invading other organs.
What allows the migrating epithelial sheet to remain as a unit? It is now understood that epithelial cells use tension-pulling force-arising at the cell-cell interface to communicate with each other. As the first author Kenji Matsuzawa explains, “Imagine a state of constant tug of war at the borders around a cell. When one cell pulls on another, that cell recognizes that it's being pulled and it pulls back. These processes are called force sensing-the recognition of being pulled-and force transmission-the pulling back. And this allows the epithelial sheet to establish a force equilibrium to prevent the individual cells from pulling apart.”
“But in the case of a migrating sheet, the cells at the front have to tell the cells rearward, ‘Hey, we're going this way’. So there's a directional element to force transmission at work here and we wondered how this directionality is passed on between cells.”
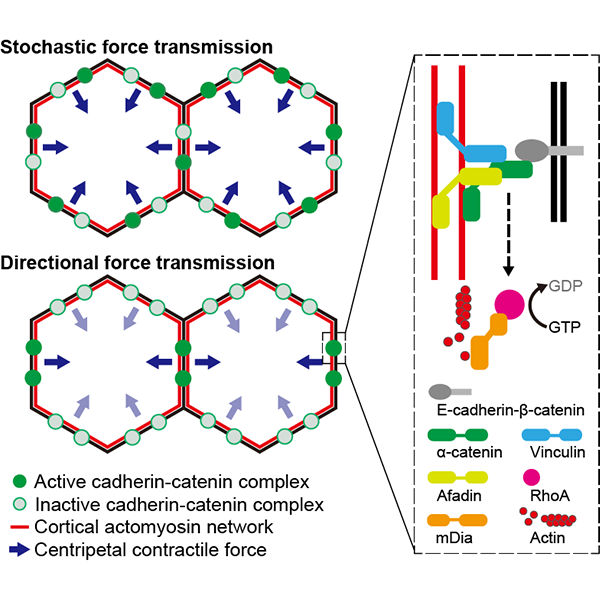
In order to address this question, the team established cells that could not sense changes in intercellular tension by altering the protein responsible for force sensing, α-catenin. “We refer to α-catenin as a ‘molecular spring’ because it stretches and contracts in response to the level of tension it experiences”, says Matsuzawa. “And when α-catenin was unable to detect tension, we found that the cell sheet no longer behaved cohesively and even neighboring cells would try to move in different directions.”
Looking deeper, the team found that α-catenin controlled the activity of the intercellular signaling pathway associated with the actin cytoskeleton, which controls cell shape and underlies the cellular response to the tension it experiences. Notes Matsuzawa, “We expected that stretched α-catenin, which is under high tension, would bind to a specific set of proteins-and we saw that. But what was really interesting was the change occurring in the cell, how once it sensed the elevated tension, the cell altered itself to communicate that information to the next cell and so on.”
Professor Ikenouchi said, “As revealed in this study, the cell adhesion complex not only offers mechanistic bonds to form epithelial cell sheets, but also plays an important role in the transmission of various information between cells. In our laboratory, we will clarify the roles of cell adhesion in the multicellular organisms through functional analysis of constituents of cell adhesion complex including membrane lipids, membrane proteins, undercoat proteins and cytoskeletons. We also would like to contribute to the elucidation of pathological conditions caused by abnormality of cell adhesion”.