
HOME / Departments / Chemistry / Photofunctional Materials Chemistry
Photofunctional Materials Chemistry
-
- SATO Osamu, Professor ◎
- KANEGAWA Shinji, Assistant Professor ◎
- ◎ Institute for Materials Chemistry and Engineering
- The development of functional molecular compounds for use in molecular electronic devices is an important challenge in the field of materials science. Our group aims to synthesize switchable materials with superior physical properties by controlling electron transfer, spin configuration, orbital movement, proton transfer, and molecular orientation in molecular crystals. In particular, we are currently focusing on the development of magnetic, conducting, ferroelectric, and mechanical materials whose properties can be controlled by external stimuli such as light and temperature. These switchable compounds can be used in future applications such as memory devices and optical switches.
1. Phototunable Magnets
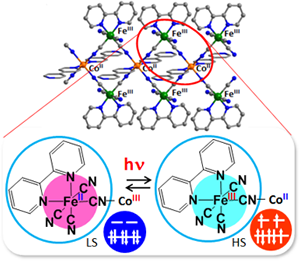
We previously reported that FeIII spin crossover and Co valence tautomeric compounds exhibited a light-induced excited spin-state trapping effect and a photoinduced long-lived valence tautomerism, respectively. In addition, we reported photoinduced magnetization effects in FeCo Prussian blue analogues and cyanide-bridged FeCo and FeFe one-dimensional compounds. Figure 1 shows the structure of the FeCo one-dimensional complex [Fe(bpy)(CN)4]2Co(4,4′-bipyridine). When a charge-transfer band in the FeCo complex was excited with 532-nm light, photoconversion from the FeII–CN–CoIII–LS to FeIII–CN–CoII–HS state was induced. Magnetization measurements revealed that the photoinduced metastable state exhibited single-chain magnetic behavior.
2. Switchable High-Spin Clusters
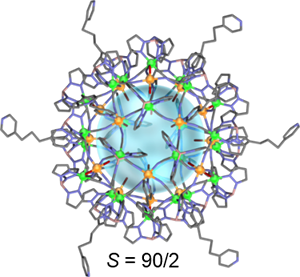
Several magnetic clusters with switching properties were synthesized. Figure 2 shows a mixed valent Fe42 (FeIII18FeII24) compound, [{Fe(Tp)(CN)3}24{Fe(H2O)2}6{Fe(dpp)(H2O)}12 (CF3SO3)6]•18H2O (Tp = hydrotris(pyrazolyl)borate, dpp = 1,3-di(4-pyridyl)propane), structured as a supramolecular cage with nanometer-sized inner space, which is the largest pore in cyano-bridged magnetic metal–organic polyhedra. Based on the properties of the magnetic material, metal centers were ferromagnetically coupled, yielding the highest ground-state spin number S = 90/2 of any prepared molecule. Furthermore, a thermal-induced charge transfer between FeII and FeIII ions upon reversible coordination bond formation/cleavage on Fe sites was observed.
3. Dynamic Mechanical Compounds
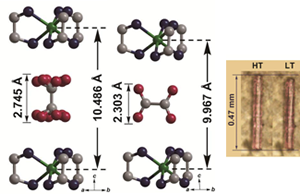
Mechanical compounds exhibiting a large crystal shape change were synthesized. The structure of the Ni complex [NiII(en)3](ox) (en = ethylenediamine; ox2− = oxalate dianion) is shown in Fig. 3. A shape change in the Ni complex was induced by an unusual 90° rotation of oxalate molecules. The oxalate dianions act as molecular-scale rotors, whose movement was propagated through the entire crystalline material via intermolecular hydrogen bonding. Consequently, a change in a sub-nanometer scale of each oxalate molecule was amplified to a micrometer-scale contraction–expansion of the crystal.