HOME / Departments / Chemistry / Nanofunctional Chemistry
Nanofunctional Chemistry
-
- YAMAUCHI Miho, Professor ◎
- ◎ Institute for Materials Chemistry and Engineering
- We synthesize nanometer-seized materials to exhibit high functionalities in catalysis, ion conduction, gas storage and magnetics for the realization of sustainable society. Electric structures, compositions and morphologies of the materials composed of alloys and oxides nanoparticles are controlled to achieve highly efficient energy and material conversions. Composite materials of porous coordination polymers demonstrate novel catalytic and ion conduction abilities through cooperative interactions among ligands and metal species. Prepared nanomaterials are applied as a device for electric power charge/discharge, catalytic materials transformation, hydrogen storage and permanent magnetism. Our final goal is elucidation of guiding principles to realize functionalities of materials and demonstration of higher functionalities based on our own principles.
1. Development of nanocatalysts for the realization of sustainable energy circulations
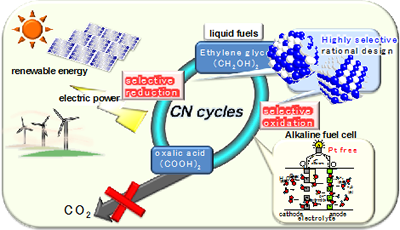
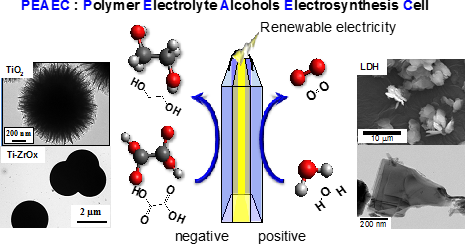
Energy carriers, which are high-energy chemicals produced using low-carbon technologies, have attracted substantial attention as storage and transportation media for renewable electricity. We have proposed novel energy cycles where electrical power is generated by partial oxidation of highly deliverable alcohols, an energy carrier, without CO2 emission, and alcohols are regenerated from oxidized wastes by using renewable energies, resulting in CO2-free power circulation, i.e., carbon-neutral (CN) energy cycles (Fig. 1). We demonstrate the successful synthesis of a well-mixed nanoalloy catalyst that exhibits selective electrooxidation of an alcohol into carboxylic acid. Renewing alcoholic compounds by direct electroreduction of carboxylic acid is another challenge. Recently, we could demonstrate electroreduction of a carboxylic acid into an alcoholic compound with significantly high selectivity using structure-controlled TiO2 catalysts. These results are the first demonstration of a CO2-free power circulation by highly selective catalysis. Currently, a polymer electrolyte alcohol electrosynthesis cell (PEAEC, Fig. 2) is developed for more efficient charge of renewable electricity.
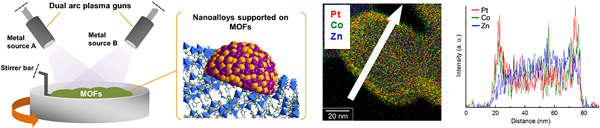
2. Functionalization of porous metal–organic frameworks (MOFs)
We offer a new approach for the facile preparation of metal-loaded MOFs (M/MOFs) through an arc plasma deposition (APD) method. We succeeded in gram-scale preparation of M/MOFs including various metal NPs such as Pt, Pd, and Ru on various MOF supports, which will be a new class of functional catalysts.