
HOME / Departments / Chemistry / Inorganic Reaction Chemistry
Inorganic Reaction Chemistry
-
- UTSUNOMIYA Satoshi, Associate Professor
- The aim of my research is to understand the fundamental processes involving nanoparticles in various environmental problems that currently occur on the Earth surface. Specifically, I am interested in the property and behavior of natural and engineered nanoparticles in the ambient environments, their interaction with biological material such as microbes and human respiratory system, and geochemical and biochemical behaviors of toxic elements including radionuclides. Research topics frequently expand to nuclear waste management and atmospheric pollution.
Visit our web site,http://www.scc.kyushu-u.ac.jp/ircl/utu-e/index-e.htm, for further information!
Research topic I
Groundwater colloids play a key role on migration of low soluble toxic elements. The process is dependent on the aggregation that can be affected by various factors including adsorbed organic compounds. Extracellular polymeric substances (EPS) derived from microorganisms are one of the important organic matters in sub-surface environment, which is typically composed of proteins, polysaccharides, nucleic acids and inorganic ions. Despite of the ubiquitous occurrence of EPS, the impact on colloid aggregation has not been explored. The objective of this study was to elucidate the effects of saccharides and phosphate on the aggregation of non-soluble nanoparticle based on the laboratory experiment.
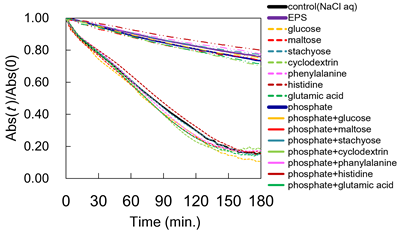
100 ppm of CeO2 nanoparticles (< 7 nm; CeNPs) were contacted with NaCl solution (1 or 10 mM; control) at pH of 6.0, adding 0.12 mM saccharide (type S), adding 0.10 mM amino acid (type A), adding 0.16 mM phosphate (type P), adding 0.12 mM saccharides and 0.16 mM phosphate (type S+P), or adding 0.10 mM amino acids and phosphate (type A+P). Four different saccharides or three different amino acids were used in the present experiment; D-glucose, D-maltose, stachyose, and α-cyclodextrin or L-histidine, L-glutamic acid, L-phenylalanine. The turbidity was analyzed by UV-vis. Solution analysis was conducted using ICP-AES and TOC. Zeta potential of CeNPs was also measured.
In the types P, S+P and A+P, the aggregation of CeNPs was inhibited, while type S and A exhibited no inhibition. The aggregation inhibition was attributed to the electrostatic repulsion by the adsorbed phosphate. The amounts of stachyose and α-cyclodextrin adsorbed on the CeNPs were reduced by 50% in the presence of phosphate, most likely because the number of adsorption sites for saccharides decreased by the adsorbed phosphate.
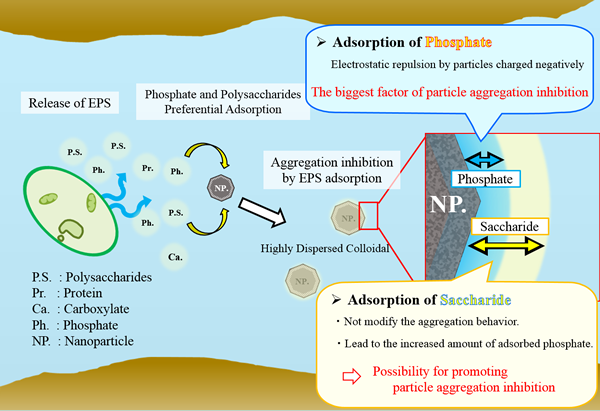
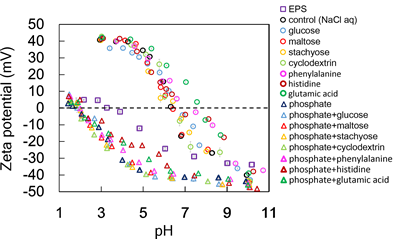
Zeta potential of the type S showed almost the same value as that of control. In the type S+P the amount of phosphate adsorbed on CeNPs was 1.1–1.5 times greater than that in the type P, despite the zeta potential of the type S+P, −40 – −41 mV, was almost same as that of the type P. In the type S+P, adsorption of saccharides lead to the increased the area of the sliding surface of CeNPs, and the electrostatic repulsive forces between CeNPs and aqueous phosphate were reduced, resulting in the increased amount of adsorbed phosphate. Consequently, phosphate in EPS inhibits nanoparticles aggregation, while saccharides do not modify the aggregation behavior but moderate the increased surface charge.
Research topic II
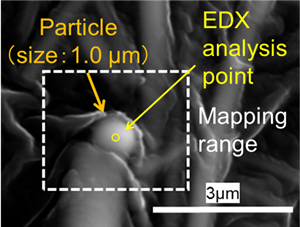
Cesium-rich micro-particles (CsMPs) retain novel information on the molten core-concrete interaction (MCCI) that happened inside the Fukushima Daiichi Nuclear Power Plant (FDNPP). In order to elucidate the micron-scale processes of radionuclides release during the explosion, systematic micro-analyses were completed on the CsMP using a variety of electron microscopy techniques.
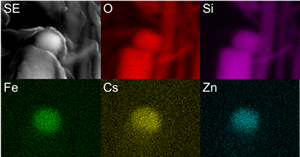
CsMPs specimens were discovered from paddy soils in Okuma town and in atmospheric particulates collected at Tokyo, by using autoradiography and SEM. Subsequent to gamma radioactivity measurement, the CsMP was thinned by using a focused ion beam system. STEM was employed for nano-scale analysis.
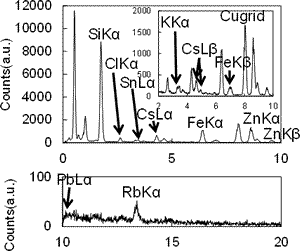
The size,134Cs and137Cs radioactivities were determined to be 0.58–5.3 µm, 0.0484–145 Bq, 0.0484–134 Bq, respectively. All CsMPs were mainly composed of Si oxide associated with Fe, Cs, Zn, Sn, Rb, K, Mn, Cl and Pb. The Cs concentration ranges from 0.81–36 wt% as Cs2O. The electron diffraction pattern revealed diffused diffraction maxima, indicating the amorphous structure. Various nanoparticles were identified at the size of 2–40 nm; crystalline Ag2Se0.5S0.5, silver telluride and Sn metal nanoparticles, indicating that a part of volatile fission products such as Te and Se associated with Ag to form airborne nanoparticles. Numerous pores were present in the center, which is the evidence of entrapped CO2 and H2O sparged during MCCI. Surface and interior of the CsMPs exhibited unique texture of nanoparticles aggregation. Hence, condensation of SiO(g) proceeded by the immediate incorporation of soluble Cs already present as vapors and entrapment of airborne nanoparticles liberated from the debris as the particle grew. Still, the CsMP is another important route of dispersion of the low-volatile radionuclides to the surrounding environment.