
HOME / Departments / Chemistry / Coordination Chemistry
Coordination Chemistry
-
- SAKAI Ken, Professor
- OZAWA Hironobu, Associate Professor
- YAMAUCHI Kosei, Associate Professor
- OKAUE Yoshihiro, Associate Professor (Lecturer)
- Zhang Xian, Assistant Professor
- Our group focuses on the realization of artificial photosynthetic molecular systems driving splitting of water (H2O) into H2 and O2 with use of solar energy (2H2O + 4hν → 2H2 + O2). With this aim, we have performed the design and synthesis of new molecular catalysts based on metal complexes that can promote both water reduction and water oxidation processes in high turnover frequencies with lower applied overpotentials. In order to realize the hydrogen society, development of such fast catalysts that can function with low overpotentials is crucial in minimizing the input energy to drive the overall water splitting processes. This approach is directed to expand the wavelength range used for solar water splitting to a wider range that extends to a lower energy region of solar spectrum. Thus, our group targets development of new molecular catalysts that drive both water oxidation and reduction with a lower applied overpotentials. For their wide spread practical use in the future, catalyst development using cheap abundant non-precious metals, such as Fe, Cu, Ni, Co, etc., is also targeted in these studies. Improvement in the photoinduced electron transfer processes that generate reducing and oxidizing equivalents from solar light absorption is also targeted.
Concept of our studies
Natural photosynthesis in green plants carries out photoinduced electron uptake from water to evolve O2 with the aid of OEC (Oxygen Evolving Center) installed in PSII. The central role of OEC is governed by the highly active catalytic performance of the tetramanganese molecular catalyst, which is known as a natural catalyst for O2 evolution. With electrons taken out from water, nature photochemically produces high-energy molecules such as NADPH and ATP, finally leading to the generation of carbohydrate via the dark reaction. In this context, our group focuses on the development of “artificial” photosynthetic molecular systems promoting the splitting of water into H2 and O2 with use of solar light based on coordination compounds as nature does. With the combustion of H2, we can uptake energy of 238 kJ/mol. In order to establish such systems, we have developed several molecular systems and devices as described below.
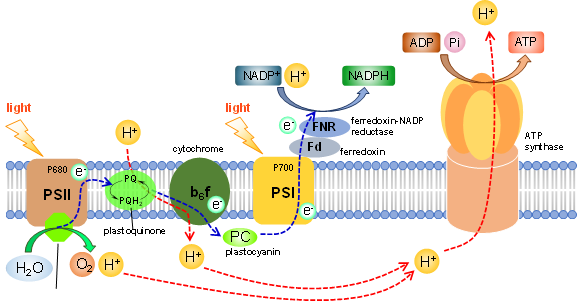
1. O2-evolving molecular systems
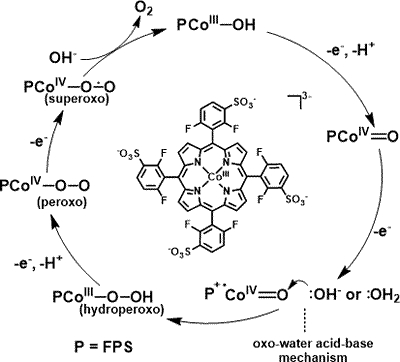
Our group has recently succeeded in inventing several new transition-metal-based molecular catalysts for water oxidation to O2. These involve mononuclear ruthenium catalysts, mono and dicobalt polyoxometalate catalysts, and Co porphyrins (see right). These together with other molecular catalysts will be thoroughly investigated to clarify the factors that govern the highly active natures of molecular catalysts for water oxidation processes and also to gain rational molecular design strategies to improve the catalytic performances of these molecular systems.
Selected publicaitons: Chem. Commun. 2016, 52, 8018; Dalton Trans. 2016, 45, 12649; ChemPlusChem 2016, 81, 1064; Chem. Eur. J. 2015, 21, 6723; Angew. Chem. Int. Ed. 2015, 54, 7981; ChemSusChem 2014, 7, 2070; Dalton Trans., 2014, 43, 12501; Chem. Commun. 2013, 49, 6325; Chem. Commun. 2012, 48, 1653; Chem. Commun. 2012, 48, 239.
2. H2-evolving molecular systems
Our group originally developed photo-H2-evolving molecular devices, which could photochemically evolve H2 from water with a single molecule. With the spectroscopic studies, the photoinduced electron transfer events were clarified in detail. We also discovered and developed various mononuclear platinum(II) complexes that drive H2 evolution from water under visible light illumination. A macrocyclic Co-NHC complex was found to promote H2 evolution from water even driven with 150 meV of driving force. The key reaction of this catalysis was found to be metal-centered PCET to generate Co(III)-H. from Co(II).
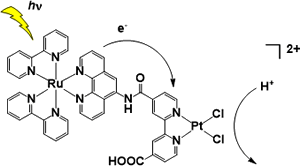

Selected Publications: Angew. Chem. Int. Ed. 2017; Nature Commun. 2016, 7, 11950; Dalton Trans. 2015, 44, 8685; Chem. Commun. 2014, 50, 9872; PCCP 2014, 16, 1607; Angew. Chem. Int Ed. 2012, 51, 7431; Chem. Commun. 2011, 47, 2227; J. Am. Chem. Soc. 2009, 131, 8404; J. Am. Chem. Soc. 2006, 128, 4926.
3. Photo-charge-separated molecular systems
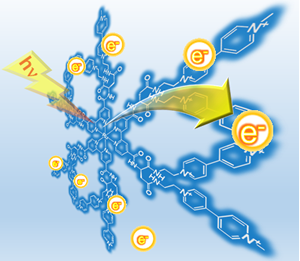
Our group also found that [Ru(bpy)3]2+-derived photosensitizers tethered to multi-electron acceptors with artificial peptide backbones. These compounds were found to show quite unusual and attractive behaviors; multi-electron storage occurs by irradiating the system in the presence of a sacrificial electron donor. Actually, this was found to be capable of charging more than two electrons over a single molecule in spite of having one photosensitizing center. Indeed, 8 electrons are stored over the framework. The special framework, 2,2′-bipyridine covalently bonded to two bis(viologen) subunits could be used to develop efficient photoinduced charge separation processes in developing efficient solar light-induced water splitting systems.
Selected Publications: Chem. Eur. J. 2016, 35, 12381; Inorg. Chem. Front., 2016, 3, 671; Chem. Commun. 2016, 52, 1385; Chem. Commun. 2015, 51, 14516; Angew. Chem. Int Ed. 2014, 53, 4618; Chem. Eur. J. 2011, 17, 1148; Dalton Trans. 2010, 39, 4421.
4. Hybrid systems (Dye-sensitized photoelectrochemical cells)
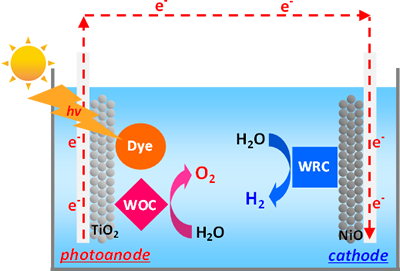
In order to achieve efficient solar-driven water-splitting reaction, hybrid systems based on coordination compounds (Dye-sensitized photoelectrochemical cells, DSPECs) have been developed in our group. DSPECs are composed of two electrodes (photoanode and cathode), and transparent conductive substrates (TCSs), such as FTO and ITO grasses, are used as substrates for these two electrodes. Generally, mesoporous TiO2 thin film, which is modified with both dye and water-oxidation catalyst (WOC), formed on the FTO glass is used as an photoanode, and mesoporous NiO thin film, which is modified with water-reduction catalyst (WRC), formed on the TCS is employed as a cathode in this system.
In our group, continuous efforts have been devoted to develop highly efficient molecule-based dyes, WOC, and WRC which possess an anchoring unit for the metal-oxide surface. In addition, preparation of photoanode and cathode, and investigation of the catalytic activity of each electrode have been so far carried out. Currently, fabrication of DSPECs and evaluation of the overall catalytic performance for the solar-driven water-splitting reaction are under way.
Selected Publications: Chem. Commun. 2017, 53, 3042; J. Mater. Chem. A 2016, 4, 1762; Inorg. Chem. 2015, 54, 8837; ACS Appl. Mater. Interfaces 2015, 7, 3152; Chem. Commun. 2015, 51, 12795; Inorg. Chem. 2014, 53, 9375; Chem. Commun. 2014, 50, 6398.