
HOME / Departments / Chemistry / Structural Chemistry
Structural Chemistry
-
- OHASHI Kazuhiko, Associate Professor
- Molecular clusters and cluster ions are useful model systems for investigating intermolecular interactions at the microscopic level. Our group has been studying structures and dynamics of molecular clusters and cluster ions through spectroscopic experiments and theoretical calculations. Currently, we focus on the interaction between metal ions and solvent molecules. Our research subjects include (1) coordination and solvation structures of transition metal ions, (2) cooperation and competition between metal ion–solvent and solvent–solvent interactions, (3) comparison of coordination numbers between ions in the gas phase and in bulk solutions, (4) temperature effects on prevalent structures of solvated metal ions, (5) interaction of metal ions with biologically relevant molecules, and so forth.
1. Spectroscopy of solvated metal ions in the gas phase
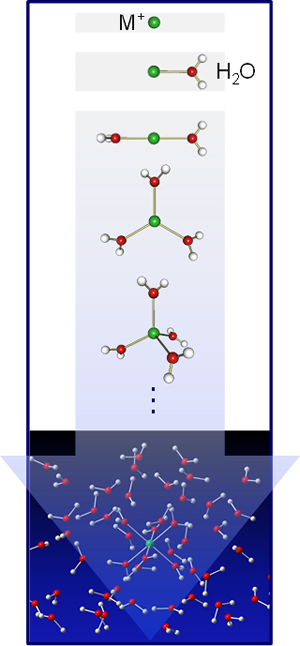
The nature of ions in solution is one of the most fundamental aspects of chemistry and biology. In particular, the hydration of metal ions is important in understanding the role of metals in the catalytic activity, the dynamics of host–guest chemistry, the structure and function of metalloenzymes, the selective ion transport through cell membranes, and so on. A variety of experimental techniques have been applied for investigating hydration structures of metal ions in solution phase. For example, x-ray and neutron diffraction experiments have been performed for determining static structures of hydrated ions. For certain metals, however, there remains ambiguity concerning the coordination number (CN), i.e. the number of water molecules bonded directly to the metal ion.
Alternatively, solvated metal ions in the gas phase have provided a unique opportunity to elucidate the ion solvation at the molecular level. In particular, infrared (IR) spectroscopy is a powerful tool for exploring the coordination and solvation structures. For instance, vibrational frequencies of the H2O molecule shift in predictable ways upon hydration. Hydrogen bonding of an OH group leads to a large low-frequency shift of its stretching vibration, whereas the vibrations of other ‘free’ OH groups are shifted only slightly. It is possible to detect the occupation of outer solvent shells through the observation of hydrogen bonding between H2O molecules.
We have investigated the coordination and solvation structures of metal ions by applying IR spectroscopy to solvated metal ions in the gas phase. Solvated metal ions of the form M+(solv)n (M = metal; solv = H2O, CH3OH, and NH3) are prepared in a laser-vaporization/super-sonic-jet cluster source. After mass selection for specifying the n value, IR spectra of M+(solv)n are measured in the OH- or NH-stretch region. Quantum chemical calculations are performed for predicting stable structures and IR spectra of M+(solv)n. What we have found are as follows.
- For solvation of a transition metal ion (M+) with small molecules, CNs are basically governed by the character of M+ rather than the solvent molecules.
- Generally, solvation of a metal ion in the gas phase gives rise to CNs smaller than those estimated from solution-phase experiments.
- Lowest-energy isomers are observed in solvated ions at low temperatures, while entropically driven isomers are preferred at higher temperatures.
2. Interaction of metal ions with bio-related molecules in solution phase
Many proteins and enzymes contain metal ions at their active sites. For example, zinc finger is a protein domain that can coordinate a zinc ion to help stabilize its structures. Cisplatin is a drug containing a platinum ion, which is used to treat many types of cancers. There is no doubt that metal–ligand interactions play important roles in these systems. In order to model such interactions, we have investigated complexes of metal ions with biologically relevant molecules through spectroscopic experiments in solution phase.
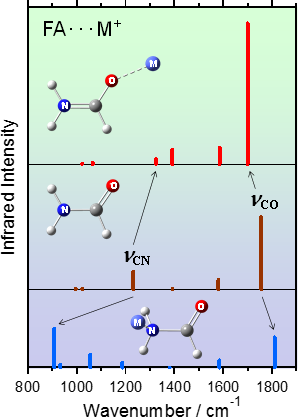
The condensation reaction of two amino acids forms a peptide bond (-OC-NH-) with expulsion of a water molecule. The peptide bond is a subject of special interest in various fields of science. Formamide (FA) is the simplest molecule containing a peptide bond. The coordination of FA to a metal ion leads to frequency shifts in the stretching vibrations of the CO and CN groups (νCO and νCN); the coordination through O atom of FA may result in a red shift of νCO and a blue shift of νCN, while the coordination through N atom may result in a blue shift of νCO and a red shift of νCN. The magnitude of these shifts is large enough to be detected by IR spectroscopy in solution phase. Our aim is to know which coordination site of FA is involved in the interaction with the metal ion and to interpret the spectroscopic observation quantitatively with the aid of theoretical calculations. The results of these studies may help us extract conformational structural information of amino acids and polypeptides in solutions.
We have applied attenuated total reflection (ATR) IR spectroscopy to neat FA and FA solutions of a series of metal ions at various concentrations. We have found that the generally believed idea about the frequency shifts of FA upon coordination is not always true.