
HOME / Departments / Chemistry / Synthetic Organic Chemistry
Synthetic Organic Chemistry
-
- UCHIDA Tatsuya†‡, Associate Professor
- † Faculty of Arts and Science
- ‡ International Institution for Carbon-Neutral Energy Research
- Organic synthetic reactions are quite important and fundamental technologies for the supplying various materials. However, most of organic transformations are consumed a lot of materials and energies with co-producing undesired waste materials. Development of highly selective, atom-economic and environment-friend new methodologies have been strongly required.
For the purpose of these viewpoints, we have intrigued to oxidative catalytic functionalization using high atom-economic oxidant, and achieved that highly enantioselective water-mediated epoxidation using air as an oxidant, and asymmetric nitrene transfer reaction using azide compound as a nitrene source.
1. High enantioselective aerobic oxidation
Molecular oxygen (O2) is an abundant, ubiquitous, and highly atom-economic oxidant on the earth. However, most of oxidation reactions with O2 as an oxidant require co-reductant for the activation of O2 and stereoselectivity are insufficient.
On the other hand, we found that Ru-salen derivatives are efficient catalyst for the asymmetric aerobic oxidative material transformations: (1) water-mediated sulfoxidation of sulfide and epoxidation of alkenes, (2) kinetic oxidative resolution of racemic sec-alcohols.
1.1 Water mediated asymmetric sulfoxidation and epoxidation

(ON)- and (H2O)Ruthenium-salen complexes could catalyze oxidation of sulfides and the epoxidation with good to high enantioselectivity using O2 as oxidant without any co-reductant.
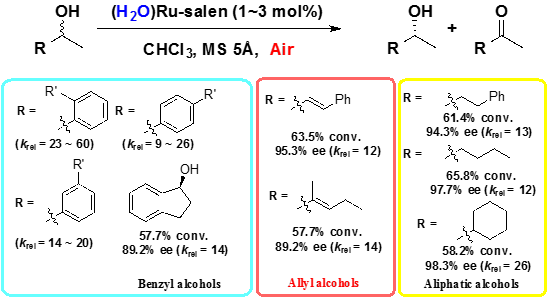
1.2 Aerobic oxidative kinetic resolution of racemic sec-alcohols
(H2O)Ruthenium-salen complex are efficient catalyst for aerobic oxidative kinetic resolution of racemic activated and unactivated sec-alcohol.
2. Asymmetric nitrene-transfer reaction
Nitrene-transfer reaction such as aziridination of alkenes, C–H amination, and imidation of sulfide are strong and straightforward tools for the nitrogen functionalization. Although, highly enantioselective nitrene transfer reactions has been studied. Most nitrene transfer reactions require the use of [(N-arylsulfonyl)imino]aryl-iodinane as the nitrene source and co-produce aryl iodide as the by-product. On the other hand, azide compounds, which give nitrene species by releasing nitrogen, are ideal precursors but rather stable. Thus, we have newly designed and synthesized (OC)ruthenium-salen catalysts for the nitrene transfer reaction with azide compound as the nitrene source and found that (OC)ruthenium-salen complexes are efficient catalyst for the asymmetric nitrene transfer reaction using azide compound under ambient conditions.
2.1 Asymmetric aziridination
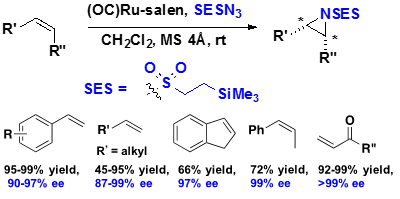
(OC)Ruthenium-salen-catalyzed aziridination can convert various olefins, even electron-deficient olefins, to the corresponding aziridine with high enantio-selectivity (up to 99% ee).
2.2 Asymmetric C–H amination

(OC)Ruthenium-salen also showed efficient catalysis in C–H amination using azide compound. The reaction only converted methylene C–H bond on ethyl group at benzylic and allylic position.