
HOME / Departments / Chemistry / Soft Interfacial Chemistry
Soft Interfacial Chemistry
-
- TAKIUE Takanori†, Professor
- † Faculty of Arts and Science
- The soft interface such as gas/liquid and liquid/liquid interfaces is regarded as a fundamental structure of soft matters including emulsion, vesicle, biological membrane, and thus the study on the structure and property of soft interface is crucial to understand sophisticated structure-function relation of soft matter. Our group investigates the adsorbed films (Gibbs films) of various surface active substances at soft interfaces by means of macroscopic and microscopic techniques; interfacial tensiometry, synchrotron X-ray reflection (XR), total reflection XAFS, and Brewster angle microscopy (BAM). Our goal is to elucidate the principles of “raft” formation in biological membrane from the viewpoint of “line tension”, which is an excess energy generated at the domain boundary in heterogeneous structure at the interface. Our recent activities are as follows
1. Phase transition and molecular miscibility in the adsorbed film and aggregates
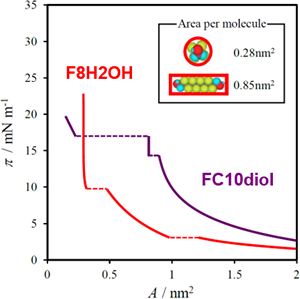
The thermodynamic analysis of interfacial tension data is used to quantitatively discuss phase transition phenomena and molecular miscibility in adsorbed films at A/W and O/W interfaces, based on the construction of 2D phase diagram (phase diagram of adsorption) and calculation of excess thermodynamic quantities. The formation of aggregates in aqueous solution including micelles and vesicles and molecular miscibility in the aggregates are also explored thermodynamically.

J. Phys. Chem. B, 118, 12451–12461 (2014)
2. Heterogeneity of soft interfacial films
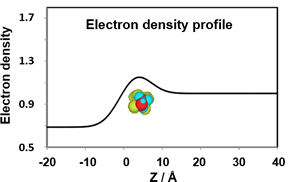
We are currently evaluating the structure of adsorbed monolayers and lipid bilayers by microscopic observation (fluorescence microscope and Brewster angle microscope), X-ray scattering measurements (X-ray reflection and small angle X-ray scattering), and fluorescence measurements (FRET). Recent studies have investigated the relationship between the heterogeneous morphology of interfacial films (2D phase separation) and the line tension generated at the domain boundary. It was found that the heterogeneous molecular distribution at the interface and differences in elasticity of the interfacial film affect the line tension pf pN order, and that a decrease in line tension causes the formation of small domains on the order of nm. These results can serve as a model for “biomembrane rafts”.